Study Purpose and Population
Epidemiology of rare diseases is important for clinicians to consider, as prevalence and incidence inform the healthcare community about likelihood of disease within an at-risk population. Studies in epidemiology also help to support economic modeling in early-stage payer communications.
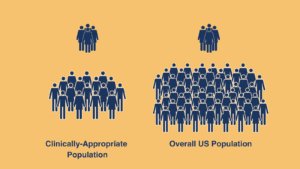
However, one of the biggest considerations when designing an epidemiologic study in rare disease is the patient population. Not all patients in the United States are at equal risk for disease, especially when a disease is rare and heritable. There may undiagnosed or underdiagnosed patients with a disease that was inherited.
Are you considering alternative denominators for a given patient population?
Real-World Example: Dravet Syndrome
Dravet Syndrome is rare and hereditary, presenting in young children who experience seizures. It is a severe form of epilepsy with frequent, prolonged seizures as the primary manifestation.
If an epidemiology study is underway to calculate prevalence and incidence, what is the total population or denominator?
Are all US patients at equal risk for Dravet Syndrome?
Without focus and attention to these questions, the goal of the study and consequential narrative may be lost, making it challenging for:
- clinicians to determine actual patients within their practice who may truly be at risk and in need of further testing
- payers who may be misinformed
- policy makers who may not understand the cascade of implications
Study Design
STATinMED uses real-world data (RWD) to assess patient populations and epidemiologic denominators that include patients who are most at risk. We find the patients who may not be diagnosed or misdiagnosed. With the example of Dravet Syndrome, we use RWD to generate evidence and insights about pediatric patients with seizures and epilepsy.
In designing a study, we discover more patients in need and share rich insights that provide clinicians with signals to prompt action for adequate diagnostics. These efforts also help when communicating with payers, highlighting the importance and urgency for timely, appropriate diagnosis and treatment. There may be 20-30 thousand pediatric patients with epilepsy covered on their plan vs communicating 1 in 20 thousand of the overall US population, since we know clinically not all of the US population is at risk of getting this disease.
Payers and Policy Makers
It is expensive to test everyone, and payers may be reluctant to cover based on findings of a study including the entire US population, however a more targeted study design may be justification for test coverage. The request is not to pay for all 10 million patients who are referred to as “covered lives” but only for a smaller segment which may be 10 thousand in the “at-risk” population.
In addition, many patients with rare disease have Medicaid as a primary or secondary coverage. For policy makers, to get payers on board with covering these diagnostics and preventive care, having the support of Centers for Medicare & Medicaid Services (CMS) to drive this forward and mandate coverage will be a more effective approach. Epidemiology models and value communications must demonstrate CMS guidelines of “reasonable but necessary”. Testing can be reserved for patients with symptoms and likely the population in which true patients are hidden or misdiagnosed.
Recommendations
We explore the vast healthcare ecosystem with STATinMED RWD Insights, our robust, licensed, all-payer medical and pharmacy claims data, sourced directly from claims clearinghouses managing transactions between payers and providers. Updated monthly with data from all 50 states encompassing over 300 million patient lives, we have current, relevant RWD with details about Medicare Fee-for-Service, Medicare Advantage, Medicaid, and commercial insurance coverage.
Often our findings demonstrate the urgency for more attention in rare disease areas which have historically been forgotten, and support discussions with payers and policy makers about the importance of covering the more expensive genomics, diagnostic tests and treatments considered to “experimental.”
We identify the most appropriate denominator populations in epidemiologic study design, to ensure clear communications with clinicians and robust information for early-stage economic models.
About STATinMED Research
Get to know STATinMED. We provide custom-built solutions by listening to research needs, identifying the best data, determining feasibility, conducting analysis and communicating value. Our data advisory services have helped clients develop the ideal data lake, sourced with curated data, and aligned with research goals.
Keshia Maughn, MPH, has in-depth skills and experience in managing outcomes research projects across therapeutic areas including oncology, immunology, women’s health, neurology, and endocrinology. With over 10 years of experience in the application of real-world evidence for advanced analytics projects, Ms. Maughn has led a global research team in HEOR and Market Access engagements. She has vast experience in econometrics and supervised machine learning techniques on a variety of data sources including open and closed network claims data, EHR data, laboratory, and genomic data. Ms. Maughn is a subject matter expert in the methodology design and the application of machine learning to provide the evidence necessary to support value communications.
We understand real-world evidence and solve problems with confidence. Contact STATinMED’s Strategic Advisory Services to learn more!
As real-world evidence authorities, STATinMED Research discovers data, develops insights and delivers optimal solutions to help life science companies realize maximum value.





